There exist numerous types of physical processes. They involve interactions and transformations of matter and require energy as well as certain conditions, such as pressure, density or temperature, in order to take place. Some may include changes in chemical composition. While some are reversible, some are not. The list below provides a short description of some of these processes.
Coalescence is the process by which two or more droplets, bubbles, or particles merge during contact to form a single daughter droplet, bubble, or particle. Coalescence manifests itself from a microscopic scale in meteorology to a macroscopic scale in astrophysics. For example, it is seen in the formation of raindrops as well as planetary and star formation.
In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. Nucleation is usually a stochastic (random) process, so even in two identical systems nucleation will occur at different times.
In molecular biology, protein aggregation is a phenomenon in which intrinsically-disordered or mis-folded proteins aggregate (i.e., accumulate and clump together) either intra- or extracellularly.
Deposition is the phase transition in which gas transforms into solid without passing through the liquid phase. Deposition is a thermodynamic process. The reverse of deposition is sublimation and hence sometimes deposition is called desublimation.
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization.
Plasma recombination is a process by which positive ions of a plasma capture a free (energetic) electron and combine with electrons or negative ions to form new neutral atoms (gas). The process of recombination can be described as the reverse of ionization, whereby conditions allow the plasma to evert to a gas.
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state.
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point.
Crystallization is the process by which solids form, where the atoms or molecules are highly organized into a structure known as a crystal.
Vitrification. Certain materials, such as glass and glycerol, may harden without crystallizing; these are called amorphous solids. Because vitrification is a non-equilibrium process, it does not qualify as freezing, which requires an equilibrium between the crystalline and liquid state.
Physical processes and phenomena obey Conservation Laws which constrain them:
- Energy: Total energy is conserved (1st law of thermodynamics).
- Momentum: Total momentum is conserved in isolated systems.
- Charge: Net electric charge is conserved.
- Angular Momentum: Total angular momentum is conserved.
However, there is one key component that is missing here. Information. Physical processes imply transfer of energy, mass, charge, or momentum. And information. Whenever energy is transformed, or transported, information is inevitably involved. And yet, when we speak of physical processes, information flow is never mentioned. One could imagine, for example, that there is a correlation between the amount of energy involved in a process and the amount of information created (or destroyed) and transferred. This correlation is quite easy to establish. It is sufficient to measure both energy and information with a certain frequency and plot the result.
Physical processes involve transformation of entropy (disorder) to structure and vice-versa. The fundamental equation of the QCT (Quantitative Complexity Theory) reflects this via the following equation:
The scalar C – complexity – captures the intensity of these transformations and quantifies the amount of information being transferred. In a system of N particles, or agents, the total amount of information at a given instant in time is:
where the first term is simply the sum of agent Shannon entropies and the second term quantifies the information encoded in structure. This is the augmented Shannon’s equation (term coined by J. Marczyk in 2025).
A new classification of physical processes (this includes biological processes) is:
- isomorphic – the structural term in C=f(S; E) is constant (dS/dt=0), more
- isoentropic – the entropy term in C=f(S; E) is constant (dE/dt=0)
- isocomplex – complexity is constant (dC/dt=0), more
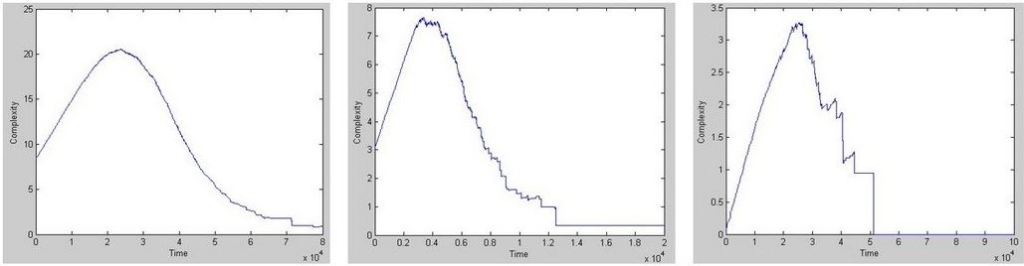
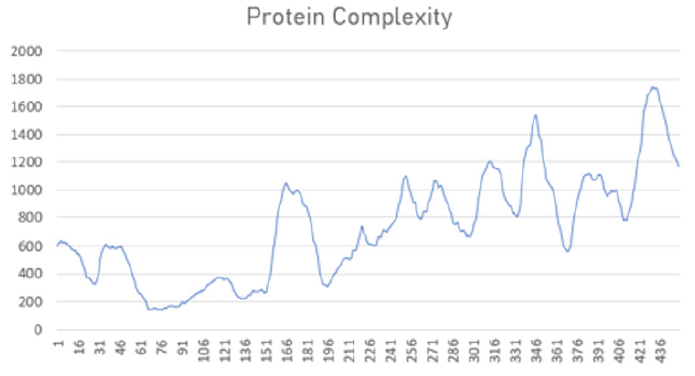
This new classification of physical phenomena can lead to new insights into the role of information transfer. A particularly interesting application is that of biological processes, as hinted by M. Vopson in his paper on infodynamics. When studying protein folding, for example, we have noticed how a protein, in its native state, maximizes the C=f(S; E) term. This makes sense because the protein ‘tries’ to encode as much information in its structure as possible, but minimising energy at the same time. An example of the evolution of complexity (information encoded in its structure) is shown below.
This is intuitively correct as without structure there is no functionality. Not surprisingly, in our biosphere there is a drive towards organisms of higher complexity (higher functionality, i.e. with more structure) and higher robustness (fitness). This drive is called co-evolution.
Example of complexity evolution in simulated biological systems that age and die in a natural (left) and traumatic (right) way.
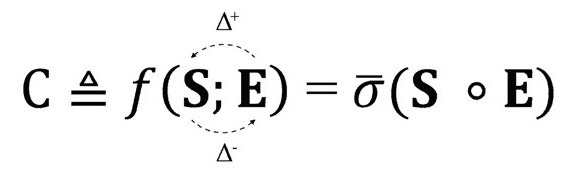
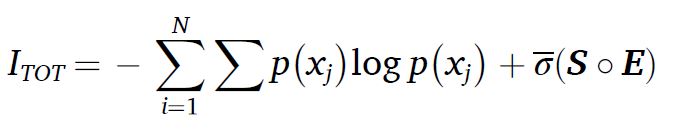
Pingback: Physical Processes and Structure <-> Disorder Transformations – Artificial Intuition