The emergence of order and structure from chaos and disorganization is a fascinating phenomenon observed in physical, chemical, and biological systems. These processes are often driven by energy input, self-organization, or the inherent properties of the system. Complexity offers a formidable way of measuring the intensity of these processes and of quantifying the amount of information involved therein.
Below are some key examples of physical processes where order and structure arise from disorder:
1. Crystallization (Liquid to Solid)
Process: A disordered liquid or solution forms an ordered, crystalline solid.
Example:
– Water freezing into ice crystals.
– Salt crystallizing from a supersaturated solution.
– Snowflakes forming from water vapor in the atmosphere.
Mechanism: Molecules arrange themselves into a repeating, ordered lattice as energy is removed.
2. Self-Assembly
Process: Disordered components spontaneously organize into structured patterns or systems.
Examples:
– Lipid Bilayers: Amphiphilic molecules (like phospholipids) self-assemble into cell membranes.
– Virus Capsids: Protein subunits assemble into highly ordered viral shells.
– Colloidal Crystals: Microparticles in a solution arrange themselves into ordered arrays.
Mechanism: Driven by intermolecular forces (e.g., van der Waals, electrostatic, or hydrophobic interactions).
3. Rayleigh-Bénard Convection
Process: A fluid heated from below develops ordered convection patterns.
Example:
– Hexagonal convection cells in a pot of heated water.
– Atmospheric convection patterns.
Mechanism: Heat causes density differences, leading to organized fluid motion.
4. Reaction-Diffusion Systems
Process: Chemical reactions and diffusion create ordered patterns.
Examples:
– Turing Patterns: Stripes or spots in animal coats (e.g., zebra stripes, leopard spots).
– Belousov-Zhabotinsky Reaction: Oscillating chemical reactions that produce spirals or waves.
Mechanism: Interaction between reaction kinetics and diffusion of reactants.
5. Magnetic Domain Formation
Process: Disordered magnetic moments align into ordered domains in ferromagnetic materials.
Example:
– Iron or nickel forming magnetic domains when exposed to a magnetic field.
Mechanism: Minimization of magnetic energy leads to alignment of spins.
6. Laser Coherence
Process: Chaotic light emissions become coherent and ordered in a laser.
Example:
– A laser beam producing highly ordered, monochromatic light.
Mechanism: Stimulated emission synchronizes photons into a coherent state.
7. Phase Separation
Process: A mixture separates into ordered phases.
Examples:
– Oil and water separating into distinct layers.
– Polymer blends forming ordered microstructures.
Mechanism: Driven by differences in solubility or intermolecular forces.
8. Biological Morphogenesis
Process: Disordered cells organize into structured tissues and organs.
Examples:
– Embryonic development.
– Formation of bacterial colonies.
Mechanism: Genetic programming and chemical signaling guide self-organization.
9. Sand Dune Formation
Process: Wind-blown sand particles organize into ordered dune structures.
Example:
– Crescent-shaped barchan dunes in deserts.
Mechanism: Wind and gravity drive the arrangement of sand grains.
10. Superconductivity
Process: Electrons in a disordered metal form ordered Cooper pairs at low temperatures.
Example:
– Superconductors exhibiting zero electrical resistance.
Mechanism: Quantum mechanical interactions lead to ordered electron pairing.
11. Cosmic Structure Formation
Process: Disordered matter in the early universe forms galaxies and stars.
Example:
– The formation of spiral galaxies like the Milky Way.
Mechanism: Gravitational collapse and dark matter interactions.
12. Rayleigh-Taylor Instability
Process: A heavier fluid atop a lighter one develops ordered structures as they mix.
Example:
– Mushroom clouds in nuclear explosions.
– Interstellar gas clouds forming stars.
Mechanism: Density differences drive the formation of ordered patterns.
13. Bénard-Marangoni Convection
Process: Surface tension gradients create ordered convection patterns in thin liquid layers.
Example:
– Hexagonal cells in evaporating liquids.
Mechanism: Surface tension and temperature gradients drive the process.
14. Polymer Crystallization
Process: Disordered polymer chains fold into ordered crystalline regions.
Example:
– Polyethylene forming crystalline domains.
Mechanism: Chain folding driven by cooling or stretching.
15. Pattern Formation in Non-Equilibrium Systems
Process: Systems far from equilibrium develop ordered structures.
Examples:
– Snowflakes: Unique, ordered patterns form as water vapor freezes.
– Liesegang Rings: Periodic precipitation patterns in gels.
Mechanism: Non-linear dynamics and feedback loops create order.
Key Concepts in Order from Chaos
– Self-Organization: Systems spontaneously organize without external direction.
– Emergence: Complex structures arise from simple interactions.
– Non-Equilibrium Thermodynamics: Order often emerges in systems driven by energy flows.
– Symmetry Breaking: A system transitions from a disordered, symmetric state to an ordered, asymmetric state.
These processes illustrate how order can emerge from chaos in nature, often driven by energy, interactions, or inherent system properties.
Transition from structure to chaos
The transition from structure to chaos is a fundamental concept in physics, chemistry, and biology, often driven by changes in energy, external forces, or system dynamics. This transition is studied in fields like nonlinear dynamics, thermodynamics, and complex systems theory. Below are key examples and mechanisms where ordered structures break down into chaos or disorder:
1. Melting (Solid to Liquid)
Process: An ordered crystalline solid loses its structure and becomes a disordered liquid.
Example:
– Ice melting into water.
– Metals melting in a furnace.
Mechanism: Heat energy disrupts the ordered lattice structure, allowing molecules to move freely.
2. Evaporation (Liquid to Gas)
Process: A liquid turns into a disordered gas.
Example:
– Water boiling into steam.
– Alcohol evaporating at room temperature.
Mechanism: Heat energy overcomes intermolecular forces, causing molecules to disperse.
3. Sublimation (Solid to Gas)
Process: A solid transitions directly into a gas without passing through the liquid phase.
Example:
– Dry ice (solid CO₂) sublimating into gas.
– Snow or ice sublimating in cold, dry air.
Mechanism: Molecules gain enough energy to break free from the solid lattice.
4. Dissolution (Solid to Solution)
Process: An ordered solid dissolves into a disordered solution.
Example:
– Salt dissolving in water.
– Sugar dissolving in tea.
Mechanism: Solvent molecules disrupt the solid’s ordered structure.
5. Phase Transitions in Non-Equilibrium Systems
Process: Ordered systems become chaotic under external stress or energy input.
Examples:
– Turbulence in Fluids: Laminar (ordered) flow breaks into chaotic turbulence.
– Plasma Instabilities: Ordered plasma structures become chaotic under high energy.
Mechanism: Energy input disrupts the system’s equilibrium.
6. Fracture and Fragmentation
Process: A solid structure breaks into disordered fragments.
Examples:
– Glass shattering.
– Rocks breaking under stress.
Mechanism: External force exceeds the material’s strength, causing it to fracture.
7. Entropy and Thermodynamic Disorder
Process: Systems naturally evolve toward higher entropy (disorder).
Examples:
– Gas expanding into a vacuum.
– Heat spreading uniformly in a system.
Mechanism: The Second Law of Thermodynamics drives systems toward maximum entropy.
8. Chaotic Dynamics in Nonlinear Systems
Process: Ordered systems exhibit chaotic behavior under certain conditions.
Examples:
– Double Pendulum: Small changes in initial conditions lead to unpredictable motion.
– Weather Systems: Ordered atmospheric patterns break into chaotic weather.
Mechanism: Sensitivity to initial conditions and nonlinear interactions.
9. Biological Degradation
Process: Ordered biological structures break down into chaos.
Examples:
– Decomposition of organic matter.
– Protein denaturation (loss of structure due to heat or chemicals).
Mechanism: Disruption of bonds and interactions that maintain structure.
10. Cosmic Disintegration
Process: Ordered cosmic structures break down over time.
Examples:
– Stars exploding into supernovae.
– Galaxies colliding and losing their structure.
Mechanism: Gravitational forces, nuclear reactions, or collisions disrupt order.
11. Chemical Reactions
Process: Ordered reactants break down into disordered products.
Examples:
– Combustion: Ordered hydrocarbons break into CO₂, H₂O, and heat.
– Explosions: Rapid release of energy disrupts ordered structures.
Mechanism: Chemical bonds break, releasing energy and creating chaos.
12. Erosion and Weathering
Process: Ordered geological structures break down into disordered sediments.
Examples:
– Rocks eroding into sand.
– Mountains weathering into valleys.
Mechanism: Wind, water, and temperature changes disrupt the structure.
13. Magnetic Domain Disruption
Process: Ordered magnetic domains become disordered.
Example:
– Heating a magnet above its Curie temperature destroys its magnetic order.
Mechanism: Thermal energy disrupts the alignment of magnetic moments.
14. Polymer Degradation
Process: Ordered polymer chains break down into smaller, disordered fragments.
Example:
– Plastic breaking down in the environment.
– Rubber degrading under UV light.
Mechanism: Chemical bonds break due to environmental factors.
15. Information Decay
Process: Ordered information becomes disordered or lost.
Examples:
– Data corruption in digital systems.
– Memory degradation in biological systems.
Mechanism: Errors, noise, or decay disrupt the ordered structure of information.
Key Concepts in Structure-to-Chaos Transitions
– Entropy: A measure of disorder, which tends to increase in isolated systems.
– Nonlinearity: Small changes can lead to large, unpredictable effects.
– Energy Dissipation: Systems lose energy, leading to disorder.
– Instability: Ordered systems become unstable under certain conditions.
These examples illustrate how ordered structures can transition into chaos or disorder through various physical, chemical, and biological processes. The formal complexity definition, shown below, illustrates the two components, order (S) and disoredr (E):
Read more in our recent blog.

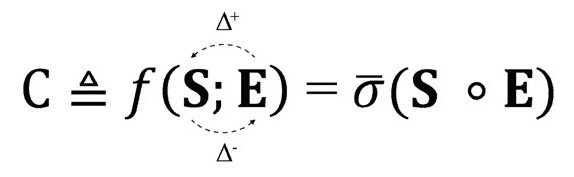
Pingback: On Blackouts, the Dynamics of Complex Systems and Post-Collapse Analysis – Artificial Intuition